Questions:
-
Explain what is the Ionization Energy (IE) and the Electron Affinity (EA) of an atom?
-
Given the following grouping of chemical elements, compare their first IE and justify:
i. Na, Cl, S, Ar
ii. Be, Ca, Ba
-
Given the value of first IE of an atom, predict if further IEs will be lower or higher and explain why. Do the same prediction for EA
-
Given the following two pairs of data:
i. (Mg, P, Na) and 1st IE/kJ mol- (1013, 496, 738);
ii. (F, I, Br) and 1st EA/kJ mol- (-324, -295, 328).
For each pair of data, match each element with the appropriate IE and EA respectively and justify your answer. -
Explain what is the Effective Nuclear Charge and illustrate by examples
Solution:
1.
Ionization Energy (IE)of an atom is the Energy required to remove an electron from an isolated atom in gaseous state and form a positive ion.:
X(g) → X+(g) + e
In other words, IE indicates how strongly an atom in gaseous state holds its valence electrons.
It is expressed in Electron-volts or Joules per mole, i.e. the amount of energy needed to ionize 1 mole of gaseous atoms. It is also called the Ionization Potential. It has a positive value (energy must be supplied). The energy to remove the first electron from a neutral atom is called the first ionization energy or potential.
Metals, due to their bigger atomic radius compared to non-metals, have low IEs compared to non-metals and this explains why metals lose electrons easily whereas non-metals lose their electrons less easily. The table below shows the trend of IE in the periodic table.
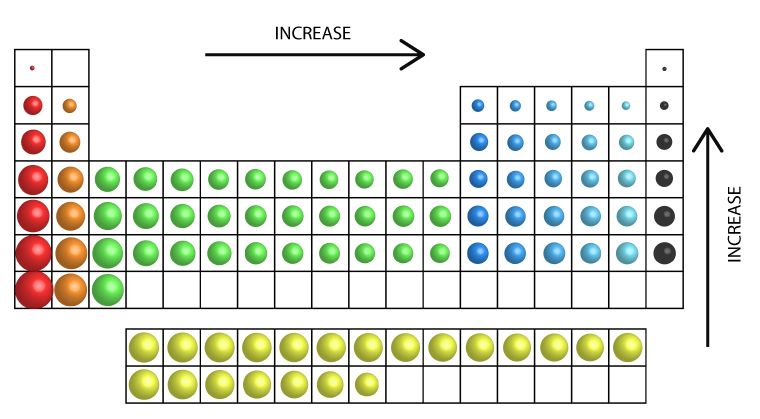
General Trend of Ionization Energy (source:surfguppy.com)
Electron Affinity (EA) of an atom is the energy change occurring when an isolated gaseous atom gains an electron to form a negative ion:
X(g) + e → X-(g)
In other words, EA indicates how an atom, in gaseous state, can capture an additional electron. The first EA of atoms is negative, meaning the attachment of the first electron to the neutral atom releases energy. But there are two exceptions to this:
(i) In group 2: Be and Mg have EA ≥ 0; Ca (-2.4); Sr (-5.0); Ba (-14.0). This may be explained by the fact that their last orbital ns2 is full, giving to the atom more stability than adding an electron in 3p orbitals.
(ii) In group 18: EA ≥ 0. This is explained by the fact that rare gases have the most stable electron configuration. Adding more electrons is not favored, hence energy must be supplied to force electron in an already stable structure.
EA is also expressed in electron-volts or joules per mole.
The table below shows the general trend of 1st EA in the periodic table.
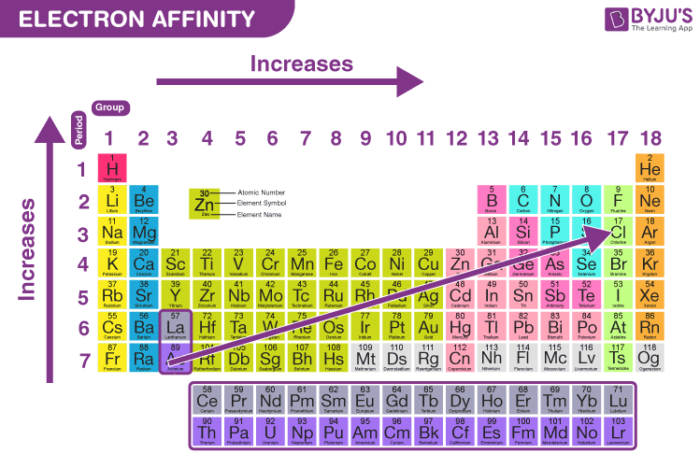
General Trend of First Electron Affinity (source:byjus.com)
(N.B: Remember that change of energy with a negative sign, -ΔE, corresponds to a release of energy, whereas change of energy with a positive sign, +ΔE, corresponds to a supply of energy)
2.
(i) Na, Cl, S, Ar
First Ionization Energy (Energy is supplied): Na < S < Cl < Ar. The atomic radius decreases from left to right in a period, hence it becomes more difficult to remove an electron from left to right due to increasing of attraction force of the nucleus towards electrons from left to right in a period.
(ii) Be, Ca, Ba:
First Ionization Energy (Energy is supplied): Be > Ca > Ba. As the atomic radius and volume increases down the group, the outermost electron is less attracted by the nucleus, hence easier to remove down the group.
3.
The first ionization leaves a cation with less number of electrons but with the same positive charge of the nucleus; this results in the reduction of the radius of the cation compared to the original atom radius before ionization. This results also in a stronger attraction of nucleus towards the remaining electrons. Therefore, further ionizations will require more and more energy than the first ionization.
Although the first EA is negative for the reaction X(g) + e → X-(g), energy released(negative value of EA), the second EA, adding an electron to the negative ion X-(g), requires energy due to the repulsion between the negative ion X- and the negative charge of the electron X-(g) + e → X2-(g) energy needed or supplied (positive value of EA). Therefore, contrary to the first EA which releases energy (-EA), further EAs will need energy (+EA).
4.
(i) Na (496kJ/mol), Mg(738kJ/mol), P(1013kJ/mol). They belong to the same period, and across a period, IE generally increases from left (metals) to right (non-metals). In this case from Na, to Mg, and then P.
(ii) This question has certainly been very difficult to answer, for one reason, an error occurred in one of the data points provided! The value of one EA given as 328 kJ/mol should be -328kJ/mol!
If that error is corrected, you may have corrected that through your research and documentation, then considering the general trend of EA in the periodic table, you may have suggested: F(-328kJ/mol), Br(-324), I(-295)
N.B: Although we should expect a decreasing trend of EA down the group 17, we have an exception. The EAs in that group are as follows: F(-328), Cl(-349), Br(-324) and I(-295).
As you can see, the decreasing trend is respected from Cl to I, however F has an exceptionally low EA, i.e. releases less energy than expected! Due to its small volume, we should expect that its nucleus would attract more strongly the incoming electron, better than the more voluminous Cl, to form F- ion. But because of that small volume, electrons in F are too crowded in that small volume and exert a stronger repulsion against the additional incoming electron, and this results in lowering the energy released by 1st EA compared to the expected one or to Cl.
5.
The Effective Nuclear Charge is the net positive charge experienced by an electron through attraction. This depends on three main factors; the positive charge of the nucleus, the distance between the nucleus and the electron, and the shielding effect from inner shell electrons (if any):
(i) Positive charge of the nucleus: the attraction force between two opposite charges is proportional to the product of those two charges. Therefore more charge more electrostatic force.
(ii) Distance between the nucleus and the electron: the electrostatic attraction force between two opposite charges is inversely proportional to the square of the distance between the two charges. Hence, longer distance, less attraction force.
F = k (q1 x q2)/r2 Coulomb law
where k = Coulomb constant, r = distance between the two charges q1 and q2
(iii) Shielding effect from inner shell electrons: apart from electrons on the first level, n =1, that experience directly the attraction from the nucleus, electrons on other levels, n =2, 3..., experience less attraction due to the shielding from the nucleus by inner shell electrons. In other words, as n increases, the shielding effect increases and the electrons experience less attraction from the nucleus. Therefore, bigger atomic volume, less effective nuclear attraction towards the outermost electrons, and small atomic volume, more effective nuclear attraction towards the outermost electrons.
Generally, the effective nuclear charge increases from left to right in a period, and it decreases down a group. This explains the trends of IE and EA in the periods and groups of the periodic table.
The effective nuclear charge affects many atomic properties such as ionization energy, electron affinity, and electronegativity (to be seen latter).