Questions:
1. In a brief historical development, explain the origin or reasons that led to the creation of the periodic table of chemical elements.
2. Explain which principle(s) or criteria the modern periodic table of chemical elements is built on, and its subdivisions.
3. What’s the importance, for Chemists, of the periodic table of chemical elements?
Solution:
Introduction
Presently (April 2021), there are 118 chemical elements known. Among those elements, most occur in nature and few are artificially produced in laboratories.
Natural elements: 94 elements (Z =1 to Z =94) occur in nature.
- Some elements occur in nature as free elements, for example, Helium (He), Hydrogen (H2), Oxygen (O2), Nitrogen (N2), Gold (Au).
- Others occur in nature in combination with other elements, for example, Tin (Sn) in a mineral called Cassiterite (SnO4), Sodium (Na) in Sodium Chloride from Sea and Ocean Water, Iron (Fe) in Haematite (Fe2O3) or Magnetite (Fe3O4), Hydrogen and Oxygen in Water (H2O).
Artificial or Synthetic elements: 24 elements (Z = 95 to Z = 118) do not occur in nature, they are synthetized in research laboratories.
1. Historical background of the periodic table of chemical elements
At the beginning of 19th century, about 60 chemical elements have been discovered. Some showed resemblances, others showed very different behaviors and properties. There was a need to have a systematic classification of those elements and their chemical and physical properties. A certain number of pioneers tried to find a solution and the most known are the Russian Dimitri Mendeleev (1869), John Newlands (1863), and Lothar Meyer (1864).
In 1869-1870, Dmitri Mendeleev and Lothar Meyer of Germany independently arrangedthe elements in order of increasing atomic weights, they observed that the elements in families appeared at regular (periodic) intervals. Mendeleev expressed this periodicity of properties of elements by stating the periodic law which states that the properties of the elements are a periodic function of their atomic weights.
But later on, after the discovery of the composition and structure of the atom, a better property or factor to ordering conveniently the elements in the Periodic Table was found to be the atomic number.
Therefore, the modern periodic law states that the physical and chemical properties of elements are periodic functions of their atomic numbers.
2. Modern Periodic Table of Chemical Elements
All known chemical elements can be found arranged in the periodic table of chemical eElements organized in 18 Groups (Columns) and 7 Periods (Rows).
A period in the periodic table is a row of elements whose atomic number increases from left to right by one unit from one element to the next.
Examples of Period 2: 3Li, 4Be, 5B, 6C, 7N, 8O, 9F, 10Ne
In a Period, each element after another is adding one electron in the same outermost shell of electrons. In Period 2, the outermost shell of electrons is 2 (n=2). Since the electrons are being filled on the same shell and at the same time the positive charge of the nucleus is increasing, this results in a stronger attraction of the nucleus towards the outermost electrons and this leads to a general decrease of the atomic radius across the Period from left to right.
In a given period, elements change from metals to non-metals from left to right, as in the example above: Li and Be are metals, B is semi-metal, C, N, O, and F are nonmetals, Ne is neither a metal nor a non-metal.
A group in the periodic table is a column occupied by elements having the same
number of valence electrons. In a Group, from up to down, the number of shells increases by one unit from a Period to the next. The result of the increasing of shells down the group is generally an increase of atomic radius down the group.
Examples of Group 2: 4Be, 12Mg, 20Ca, 38Sr, 56Ba, 88Ra
All Group 2 Elements have 2 valence Electrons, and this explains why they have similar chemical properties; that is why a Group is sometime said to form a Family; in our example, all the elements of the Group 2 are metals; and they react by losing the 2 valence electrons to form M2+ cations. Because of similarities of the elements in the same group, some groups have been given specific names:
- Group 1: Alkali Metals;
- Group 2:Alkali-Earth Metals;
- Group 17: Halogens;
- Group 18: Rare Gases.
The periodic table of chemical elements is also subdivided into three zones called Blocks, as shown in the Figure below.
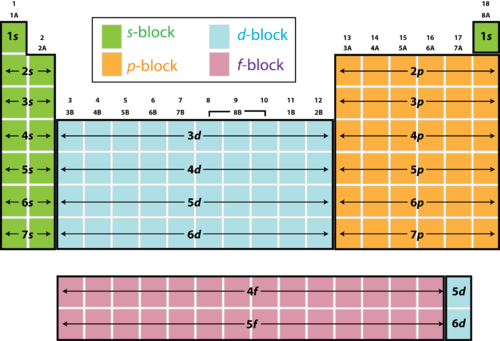
Source: courses.lumenlearning.com
s-Block (Groups 1&2): Alkali & Alkaline-Earth Metals: their valence electrons are in s orbital. They lose easily their valence electrons to form cations M+ and M++ for Group 1 and Group 2 respectively. They are very active metals, i.e. very reactive.
p-Block (Groups 13-18): their valence electrons are in p orbitals; this block is subdivided into 3 areas: the lower left area made of metals, the upper right area made of nonmetals, and a middle diagonal area between the previous ones made of semimetals or metalloids as shown in the Figure below.
s & p-Block elements are also called Main Group Elements.
d-Block (Groups 3-12): also called Transition Metals are characterized by having their valence electrons in d orbitals. They are all metals generally characterized by high melting points, except Mercury which is liquid at room temperature. They show more than one oxidation states, i.e. they form cations with different charges. Examples: Cu+, Cu2+, Fe2+, Fe3+. Transition metals are less active than Groups 1 & 2 metals.
f-Block: belong to periods 6&7 and are called inner Transition elements, with their valence electrons in f orbitals. f-block is generally considered as part of Transition Metals.
Below, another subdivision of the periodic table is shown: metals, nonmetals, and semimetals (metalloids).
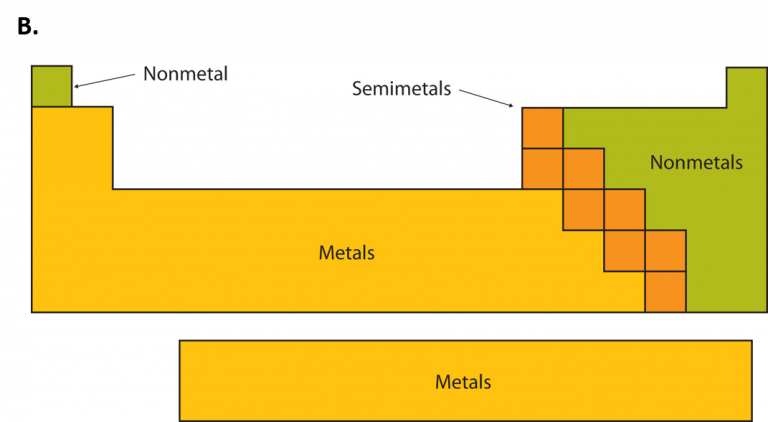
Source: wou.edu
N.B: Although hydrogen atom has its valence electron in s orbital, it behaves as metal when it combines with a nonmetal, but as a nonmetal when it combines with active metals of Group 1. That is why in many modern periodic tables, it is not put in Group 1 but is left hanging up above the other elements because of its double behavior.
3. Importance of the Periodic Table
- The periodic table can be compared to a map of a country showing different regions and their characteristics and resources.
- The periodic table gives you a general picture of all chemical elements organized in different blocks characterized by their particular properties.
- When you localize a chemical element on the periodic table, its location allows you to know its nature, and to predict its chemical and physical properties, etc.
See You Next Time!