1. Now that you know the composition of the atom. If you are to organize the electrons around the nucleus in different energy levels or orbitals, i.e. to establish the electron configuration of an atom, what are the rules and principles that are going to guide you?
2. Guided by those rules and principles, build the electron configurations of the following atoms: 5B, 14Si, 18Ar, 23V, 29Cu.
3. What are valence electrons, their role and their importance in Chemistry?
Solution:
In building the electron configuration of an atom, we must be guided by the following rules and principles:
- The Aufbau (German word for Building) Principle: this principle directs us to fill electrons on lower energy levels first, then higher energy levels; in other words, fill energy level n=1first, then n=2, then n=3, etc...
- Pauli Exclusion Principle: this principle says that no two electrons can have the 4 quantum numbers (n, m, l, s) identical. The consequence of this principle is that an orbital can accommodate a maximum of 2 electrons with opposite spins.

- The Hund’s Rule: when orbitals have the same energy, they are said to be degenerate, one electron is put in each degenerate orbital before pairing up electrons in those degenerate orbitals.
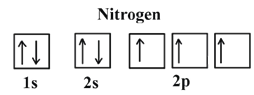
2.
5B: 1s22s22p1;
14Si: 1s22s22p63s23px1py1;
18Ar: 1s22s22p63s23p6;
23V: 1s22s22p63s23p64s23d3;
29Cu: 1s22s22p63s23p64s13d10
Attention: There are some exceptions to keep in mind when building electron configuration of an atom:
- Starting from n = 3, although the principal numbers n: 3< 4<5, etc., when building the electron configuration, 4s is filled before 3d, 5s before 4d as shown in figure below.
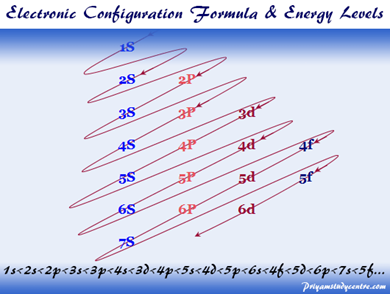
- Another exception results from stability considerations where full orbital is the most stable, followed by half-filled orbitals, as in the following electron configurations:
(a) 24Cr: 1s22s22p63s23p64s23d4 is wrong, the most stable configuration is 1s22s22p63s23p64s13d5 . It is more favorable to promote one electron from 4s2 to 3d4 and have a more stable structure of 4s13d5, where all orbitals are half-filled.
(b) 29Cu: 1s22s22p63s23p64s23d9 is wrong, the most stable configuration is 1s22s22p63s23p64s13d10, because one orbital, 4s1, is half-filled, the other, 3d10, is completely filled.
3.
Valence electrons: valence electrons are electrons in the outermost energy level of the atom. For example, valence electrons in
- Boron atom are 3 (2s22p1)
- Copper atom are 11(4s13d10)
Valence electrons are very important because they are the ones that participate in the formation of chemical bonds. In other words, they are the ones that determine the chemical properties or behavior of an atom. The other electrons, core electrons, do not participate in the formation of chemical bonds.
![Problem #6: Electron Configuration [Solved]](/content/images/size/w2000/2021/02/istockphoto-638477138-170x170.jpg)