Question: Explain the concept of Electrolytic Cell, draw a representation of it, and give concrete example of application of an Electrolytic Cell.
Solution:
(i) An Electrolytic Cell is an Electrochemical Cell in which the chemical reaction taking place in the cell is not spontaneous but must be caused by the application of an external electrical current. Such a reaction is called “Electrolysis”.
Examples:
-Electrolysis of water H2O: 2H2O(l) + electrical current = 2H2(g) + O2(g)
-Electrolysis of molten NaCl: 2NaCl + electrical current = 2Na(s) + Cl2(g)
Without the electrical current, these reactions cannot take place.
(ii) As for a galvanic cell, an Electrolytic cell has two compartments: cathode and anode (see Figure below).
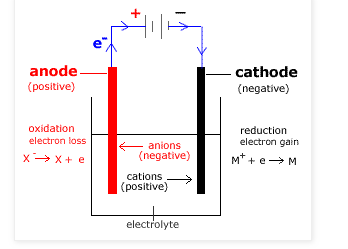
(vivadifferences.com)
The Cathode compartment (-) is where the reduction reaction takes place; it is connected to the negative pole of the external source of electrical current, source of electrons.
The Anode compartment (+) is where the oxidation reaction takes place; it is connected to the positive pole of the external source of electrical current.
In electrolysis of molten NaCl, we have the following reactions at the electrodes:
Anode (+): oxidation reaction: 2Cl- → Cl2(g) + 2e
Cathode (-):reduction reaction: 2Na+ + 2e → 2Na(l)
Overall reaction: redox reaction: 2NaCl(molten) electrolysis = 2Na(l) + Cl2(g)
For an Electrolytic cell to produce results, the external electrical current must be continuous.
Basic Electrolysis calculations
The Faraday constant
· Coulomb: a measure of the quantity of electricity.
If a current of 1 ampere (1A) flows for 1 second, then 1 coulomb of electricity has passed: 1C = 1A/sec
· The Faraday: Electricity is the flow of electrons. Each electron bears a negative charge of 1.602 x 10-19 coulomb. Therefore 1 mole of electrons (6.02 x 1023) bears: 6.02 x 1023 x 1.602 x 10-19 = 9.632 x 104= 96,320 C.
This amount of charge is known as the Faraday constant (F)
The number of charge or the quantity of electricity used is proportional to the number of moles of electrons consumed or produced at one or another electrode.
Faraday’s Laws of Electrolysis
1- First law: the weights of substances formed at an electrode during electrolysis are directly proportional to the quantity of electricity that passes through the electrode: Q = It, where Q = quantity of electricity in Coulomb; I = intensity of electrical current in ampere, and t = time in seconds
2- Second law: the weights of different substances formed by the passage of the same quantity of electricity are proportional to the equivalent weight of each substance.
Or: the charge required to deposit or liberate a mass m is given by Q = Fmz/M, where F is the Faraday constant, m the mass deposited or released at an electrode, z the number of electrons involved, and M the relative ionic mass or the molar mass of the substance deposited or released at the electrode.
From the above mathematical expression we can derive also: m = QM/zF = ItM/zF (1)
Examples of calculation:
(1) Calculate the mass of Ag deposited at the cathode during the electrolysis of AgNO3solution, if a current of 0.10A is used for 10 minutes; knowing that Arof Ag = 108
Solution:
Ag+(aq) + e → Ag(s)
1 mole of e → 1 mole of Ag
Mass (m) = Number of moles of Ag x Ar = number of moles x 108g
Quantity of electricity used: It = 0.1A x 10 minutes x 60 seconds/minute = 60 C
Number of moles Ag = It/1F = 60C/96,320 C.mole-1= 6.22 x 10-4
Mass of Ag = 6.22 x 10-4 x 108 g = 671.76 10-4g = 0.067 g = 67 mg
N.B: You can also apply directly the expression (1) above.
(2) Calculate the mass of H2 produced, at room temperature-rtp, during the electrolysis of dilute H2SO4, if you use a current of 10A for 15 minutes.
Solution:
2H+(aq) + 2e → H2(g)
1 mole of H2 requires 2 moles of e or 2 x 96,320 C = 192,640 C
Quantity of electricity used: It = 10A x 15 minutes x 60 seconds/minute = 9,000 C
Number of moles of H2 = It/2F = 9,000C/2 x 96,320C = 9,000 C/192,640 C = 0.047 mole
Mass of H2 at rtp: number of moles x 2g/mole = 0,047 mole x 2 g/mole = 0.094 g = 94 mg