Questions: 1- Explain the concept of Electrode Reduction/Oxidation Potential. 2- Explain the concept of Standard Electrode Reduction/Oxidation Potential
Answers:
1- The Potential, as a name, according to the Dictionary, means: Latent qualities or abilities that may be developed and lead to future success or usefulness.
If we use the same definition, we can say the Electrode Potential is the latent quality or ability that may be developed and lead to future usefulness of the electrode.
To explain further the concept of Electrode Potential, let’s use the following analogy.
Suppose that you have at home in the living room, a decoration object, weighing 1 Kg, suspended on the ceiling. That object has a Potential Energy, let’s call it P. If you cut the rope that holds it, it falls by itself down on the floor, it will do work (damaging the floor of heating it). In other words, the potential energy, when the object was suspended on the ceiling has changed into another form of energy (mechanical energy or heat energy); this has happened naturally.
Once on the floor, can the decoration object rise up to the ceiling? Not naturally. If you want the object back to the ceiling, you must use an external force. Contrary to the first movement of falling down of the object that could transform its potential energy, P, into work, this time energy must be supplied to bring back the object to the ceiling.
That example can be compared to the Electrode Potential.
Let’s consider a strip of Zinc metal, Zn, dipped into a solution containing Zn2+ ions. The zinc metal is called Electrode. This creates an interface or contact Zn:Zn2+, that we represent by Zn/Zn2+. Zn/Zn2+is called a redox couple. At that interface, there is a tendency of metal ions from the solution do be reduced and form metal atoms: Zn2+ + 2e → Zn This tendency is called, the Electrode Reduction Potential
At the same time, metal atoms of the electrode have tendency to go into the solution as ions and leave behind the electrons at the electrode: Zn → Zn2+ + 2e This tendency is called the Electrode Oxidation Potential
Here we see that we have two competing processes at the electrode: Reduction and Oxidation. Depending on different factors such as the nature of the electrode, the concentration of the electrolyte, reduction or oxidation reaction may be favored.
For a given M/Mn+ couple, Electrode Reduction and Oxidation Potentials are equal but of opposite signs. Example: if the Electrode reduction potential of a redox couple is V (volts), the opposite Electrode oxidation potential is –V (volts).
Therefore the Electrode Potential is the potential difference between the electrode and the solution in a half-cell.
Half-cell is an electrode in contact with a solution of its ions; example: Zn/Zn2+
2- Standard Electrode Reduction Potential
As seen above Electrode reduction potential and Electrode oxidation potential have the same values but opposite signs. In this explanation we’ll talk about Standard Electrode Reduction Potential as recommended by IUPAC, but the same principles apply for Electrode Oxidation Potential.
How do we measure the Electrode Potential?
There is no way a single Electrode of Half-cell potential can be measured. Only the difference of electrode potentials between two connected half-cells can be measured.
By International convention, Hydrogen (H2/2H+) has been taken as the reference electrode. It consists in a half-cell in which an inert metal foil is immersed in a solution of hydrogen ions, H+, and hydrogen gas is bubbled over the foil.
The Standard Hydrogen Electrode (SHE), used, in measuring Standard Electrode Potentials, uses a platinum (Pt) foil with a 1.0 M solution of hydrogen ion, H+, hydrogen gas, H2, at 1 atmosphere, at a temperature of 25oC. It is represented as: Pt(s)/H2(g), H+(aq). The reduction reaction is: 2H+(aq) + 2e → H2(g).
The conditions of 1.0 M solution and 1 atmosphere of H2 and 25oC constitute the “Standard Conditions”.
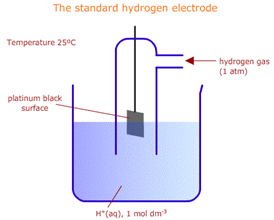
(socratic.org)
By international agreement, the Standard Hydrogen Electrode, as a reference electrode, has been given a Reduction Potential of 0 V
The Electrode Standard Reduction Potential, represented by the symbol Eo with volt units, V, of any Half-cell is the Reduction Potential difference between Standard Hydrogen Electrode Potential, 0 V, and that other Electrode in Standard Conditions, when the two electrodes are connected.
Table of Selected Standard Reduction Potentials
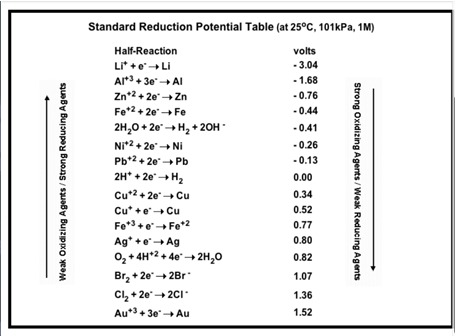
(chegg.com)
A positive value of Eo indicates that the species reduces more easily than H+ ion, whereas a negative value of Eo indicates that H+ ion reduces more easily than the species.
Examples:
- Cu+2(aq) + 2e → Cu Eo = 0.34 V
- 2H+(aq) + 2e → H2 Eo = 0 V
- Li+(aq) + e → Li Eo = -3.04 V
These examples show that Cu+2 ion is reduced to Cu metal more easily that H+ ion to H2, whereas H+ ion is reduced to H2 gas more easily than Li+ion to Li metal.
In the same way, Cu+2 ion reduces into Cu metal more easily than Li+ ion into Li metal.