Answers to the questions:
1- Describe how a volumetric titration is done.
2- Given a sample solution of HCl(aq) of unknown concentration; if 25.00 mL of that solution are neutralized by 20.45 mL of NaOH[0.10M]. What’s the concentration of HCl(aq) solution?
3- Given a sample solution of NaOH(aq) of unknown concentration; 25.00 mL of that solution are neutralized by 18.38 mL of H2SO4[0.10M]. What’s the concentration of NaOH(aq) solution?
4- Given a sample solution of Na2CO3[0.15M]. How many mL of HCl[0.8M] will be required to react exacltly with 25.00 mL of Na2CO3(aq)?
1. This technique is based on the volumes of reactants that react according to the balanced chemical equation of the reaction between two reactants.
The procedure consists in that a sample or a solution of analyte, 1, whose concentration is unknown is reacted with a known amount of a reactant 2 whose concentration is known. The reactant 2 is called, “standard solution” or “titrant”. The end of the reaction is indicated by an indicator that changes color at the end of the reaction; the end of the reaction is also called the equivalence or end point. This is procedure:
(1) A given precise volume (ex: 25.00 mL) of the sample is transferred into an Erlenmeyer, and few drops of the indicator (if necessary: the color of some reactants themselves can serve as indicator) are added (2-3 drops).
(2) The standard solution is filled in a burette fixed on a stand above the Erlenmeyer containing the sample as shown below
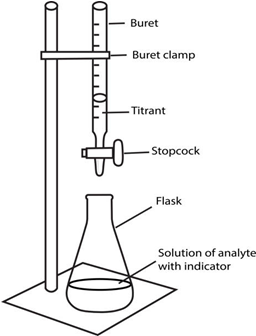
(istockphoto.com)
(3) The titrant is added slowly through the stopcock to the sample solution, swirling continually for a homogenous mixing.
(4) When change of color begins to appear and disappear, slow down adding the titrant and add drop-by-drop until the change of the color is permanent; avoid to add excess of the titrant.
(5) Write down the volume of the titrant used.
Based on the stoichiometric relationship or the mole ratio of the chemical equation, the concentration of 1 can be deduced/calculated from the amount of 2 used.
2. HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
1 mole of HCl + 1 mole of NaOH → 1 mole of NaCl(aq) + 1 mole of H2O(l)
Calculation:
(a) 20.45 mL of NaOH[0.10M] = (0.1 mole/1000 mL) x 20.45 mL = 0.002 mole
(b) 0.002 mole of NaOH react with 0.002 mole of HCl, according to the balanced chemical equation.
(c) 0.002 mole of HCl in 25.00 mL of HCl corresponds to: (0.002 mole/25.00 mL) x 1000 mL = 0.08 mole/L
Therefore the concentration of HCl (aq) solution is: 0.08M
3. H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + 2H2O(l)
1 mole of H2SO4 + 2 mole of NaOH → 1 mole Na2SO4+ 2 moles H2O
Calculation:
(a) 18.38 mL of H2SO4[0.10M] = (0,1 mole/1000 mL) x 18.38 mL = 0.0018 mole
(b) 0.0018 mole of H2SO4 react with 0.0036 mole of NaOH, according to the balanced chemical equation.
(c) 0.0036 mole of NaOH in 25.00 mL of NaOH corresponds to: (0.0036 mole/25.00 mL) x 1000 mL = 0.14 mole/L
Therefore the concentration of NaOH(aq) solution is: 0.14M
4. Na2CO3(aq) + 2HCl(aq) → 2NaCl(aq) + CO2(g) + H2O(l)
1 mole of Na2CO3 + 2 moles of HCl → 2 moles of NaCl + 1 mole of CO2 + 1 mole of H2O
Calculation:
(a) 25.00 mL of Na2CO3[0.15 M] = (0.15 mole/1000 mL) x 25.00 mL = 0.004 mole
(b) 0,004 mole of Na2CO3react with 0.008 mole of HCl, according to the balanced chemical equation
(c) 0.008 mole of HCl[0.8M] are in: (1000 mL/0.8 mole) x 0.008 mole = 10.00 mL
Therefore the volume of HCl[0.8M] required is: 10.00 mL
N.B: This is my preferred method of calculation.
But another method exists:
(i) When the mole ratio in the balanced chemical equation is 1:1 such as in the reaction:
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Based on 1:1 mole ratio of the balanced chemical equation, the following formula applies:
C1V1= C2V2
Where C1 and C2represent molar concentrations, and V1 and V2 represent the volumes of the titrant and the solution analyte respectively.
0.10 M x 20.45 mL = C2x 25.00 mL
C2= 0.10M x 20.45 mL/25.00 mL = 0.08M
(ii) If the mole ratio in the balanced equation is 1:2, as in:
H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + 2H2O(l)
2 x C1V1 = C2V2
2 x 0.10M x 18.38 mL = C2 x 25.00 mL
C2= 2 x 0.10M x 13.38 mL/25.00 mL = 0.15M