Covalent Bond
A. G.N. Lewis, an American physical chemistry is known for his discovery, in 1902, of the covalent bond. He introduced the idea known as the octet rule, where he stated that: “A molecular structure is most stable when each atom in it contains an octet of electrons in its valence shell”.
(N.B: However, this octet rule is somewhat limited in scope, because many elements don’t obey it. One example is hydrogen which can accommodate only 2 electrons in its valence shell. Elements in the third row of the periodic table and beyond can use d orbitals to hold more than eight electrons in their valence shells.)
Covalent bond is formed when two atoms combine by sharing a pair of electrons. In a covalent bond, each atom participating in the formation of the bond brings 1 unpaired electron to form a pair of electrons that constitutes the bond. In doing so, each atom acquires a more stable electronic structure of a rare gas as shown in Fig.2 and 3 for the formation of Cl2 and N2molecules. Covalent bond is formed between atoms of non-metals.
Cl. + Cl. → Cl:Cl (Formation of a covalent bond)
In this case and after combination, each chlorine atom has 8 electrons in its valence shell, the same structure as the rare gas Argon, and the new unit formed is called Molecule of chlorine (Cl2).
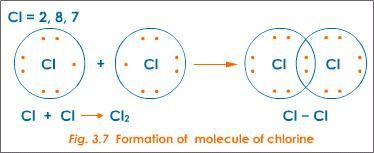
Figure 2: Formation of a covalent bond in Cl2 molecule
Source: socratic.org
When two atoms of nitrogen combine, each atom brings its 3 unpaired electrons to form 3 shared pairs of electrons between the two atoms or 3 covalent bonds between the two atoms. After the combination, each nitrogen atom acquires 8 electrons in its valence shell, and this gives N2 molecule.
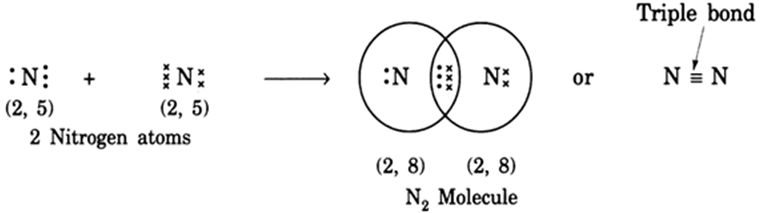
Figure 3: Formation of triple covalent bond in N2 molecule
Source:www.zigya.com
B. Lewis Diagrams or Lewis Structures
Lewis introduced also Drawing Diagrams for two purposes:
- First, to show how atoms in a compound are connected one to another,
- Second, to account for all the valence electrons of all atoms in a compound.
Molecules have different structures or shapes. Only diatomic molecules have the same shape: they are all linear. Other molecules, i.e. tri- and polyatomic molecules can have different structures, from linear to more complex structures.
A Lewis diagram or structure is a representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around each atom in a molecule. Electrons are shown as “dots” or a “line” between two atoms for bonding electrons. The goal is to obtain the “best” electron configuration, i.e. the octet structure of each atom in the molecule.
In drawing a Lewis diagram of a molecule or an ion, the following rules are followed:
- Determine the number of valence electrons of each atom in the molecular formula. When the species is an ion, each negative charge counts for 1 electron, whereas each positive charge counts for -1 electron. Then sum up the total number of electrons.
- Draw a skeletal structure for the molecule or the ion which connects all atoms using only single bonds (single line). The central atom will be the one that can form the greatest number of bonds.
- Then with the remaining electrons, add lone pairs of electrons to all atoms to complete the octet (when possible).
Hereafter are examples of Lewis structures for few molecules and ions:
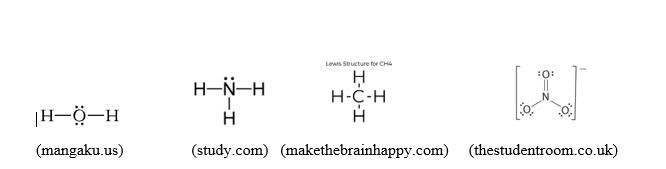
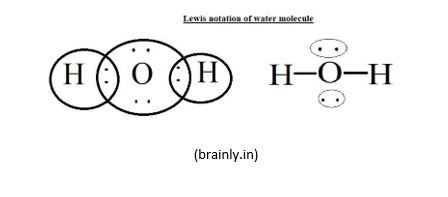
H2O:
- Number of valence electrons: 2x1 + 6 = 8
- H-O-H: 4 electrons are shared between O and the two H atoms
- The remaining 4 electrons are put on O since H atoms are already filled with 2 electrons each:
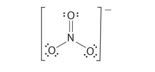
NO3-:
-
Number of valence electrons: 5 + 3x6 + 1 = 24
-
Skeletal structure:
-
The remaining 18 electrons are distributed as follows:
- 2 electrons between N and one of the 3 O atoms, i.e. a double bond N=O; this leaves N with a total of 8 electrons (octet structure achieved).
- There remains now 16 electrons to be distributed between 3 O atoms as follows:
• 2 electron pairs (4 electrons) on the oxygen double bonded with nitrogen,
• 3 electrons pairs (12 electrons) on each 2 remaining oxygen.
Although Lewis structure theory was a good starting point for the study of molecular structure, it has its own limitations:
- It couldn’t be applied to molecules such as BCl3, where the central atom B has only 6 valence electrons instead of 8, or PCl5 and SF6 where the central atom has 10 and 12 electrons respectively.
- It could not predict or explain the shapes or structures of many molecules.
- It could not explain, for example, why O2 molecule, with all electrons paired (:Ö::Ö:), is paramagnetic. Yet paramagnetism is due to the presence of unpaired electrons in the molecule!
C. Molecular Orbitals
Bonding molecular orbitals: The concept of chemical bond made a step forward with the idea advanced by Schrodinger (1926) on the existence of orbitals where electrons can be found with high probability.
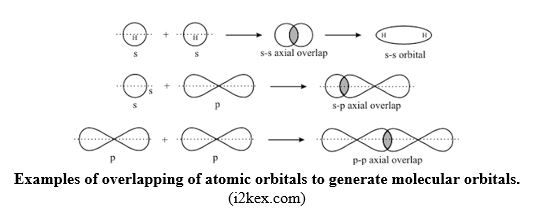
Therefore, Sharing of electrons in a covalent bond means also overlapping of atomic orbitals to generate molecular orbital which accommodates the shared electrons.
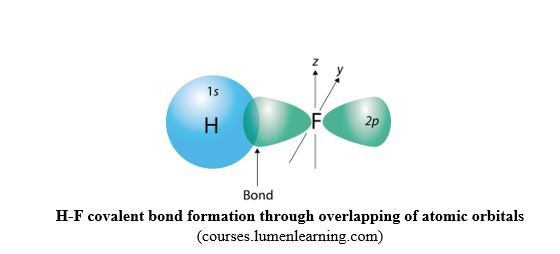
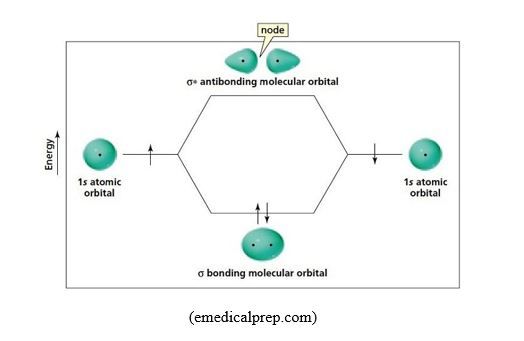
Sigma bond/σ bond: In the formation of H-F bond, atomic s orbital from H atom, with 1 electron, overlaps with atomic p orbital from F atom, with 1 electron.
When overlapping occurs by head-to-head overlapping of orbitals as in Fig.16, the result is that the cloud of the bonding electrons is in between the two nuclei, and centered on the axis joining the nuclei. Head-to-head overlapping of atomic orbitals generates a molecular orbital called σ molecular orbital, and the resulting covalent bond is called sigma bond, σ bond. Due to concentration of the density of bonding electrons on the inter-nuclei axis joining the two atoms, σ bond is strong because of a strong attraction between the bonding electrons and the nuclei.
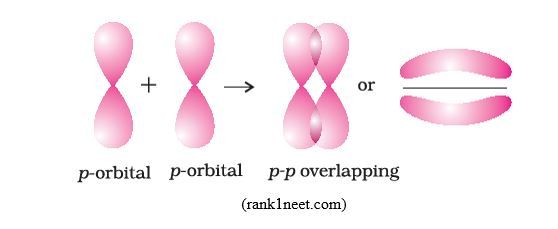
Pi bond/π bond: When overlapping of atomic orbitals takes place Side-by-side as shown below, it forms π molecular orbitals and the covalent bond created is called Pi or π bond.
As you can notice, in a π bond, contrary to a sigma bond, the cloud of bonding electrons is not on the inter-nuclei axis, it is rather in two regions located outside the axis, on both sides of the axis. In this bond, there is no binding electrons on the inter-nuclei axis and because of that, the attraction force between the nuclei and the binding electrons is weaker than in a sigma bond. In other words, π bond is weaker than σ bond; therefore, sigma bond is more stable than Pi bond.
N.B: Do not take the two orbital lobes (regions) of Pi-bond as constituting two bonds. No, they constitute one Pi-bond, characterized by two lobes separated by a nodal plane where the probability of finding electrons is zero.
D. Multiple Bonds: Double and Triple Covalent Bonds
When two atoms have each two or three electrons to share, then a double or triple bond is formed respectively:
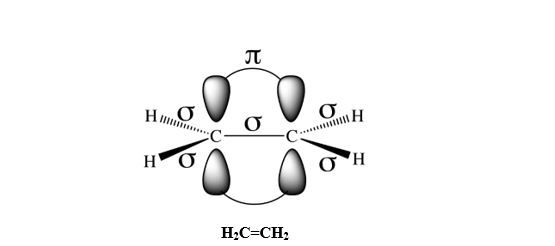
Example: Double bond: H2C=CH2; Triple bond: N≡N (Fig.2).
What happens when the combining atoms have more than one pair of electrons to share?
Take the example of N≡N. As we have seen previously, each nitrogen atom has 3 unpaired electrons to be shared as shown in Fig.2. Those electrons are in p orbitals.
For the first bond, the best overlapping is when orbitals overlap head-to-head; let’s take here px-px head-to-head overlapping. This results in the formation of one σ-bond.
Once px-px overlap head-to-head, the remaining orbitals, py-py and pz-pz, due to their orientations, overlap side-by-side to create 2 π bonds.
Notice that every time a double or triple bond is created between two atoms, one of those bonds that constitute the multiple bond, must be sigma, and the remaining Pi bond(s). A Pi bond cannot exist alone by itself; it is formed only when there are more than one pair of electrons to be shared between two atoms.
E. Electronegativity (EN)
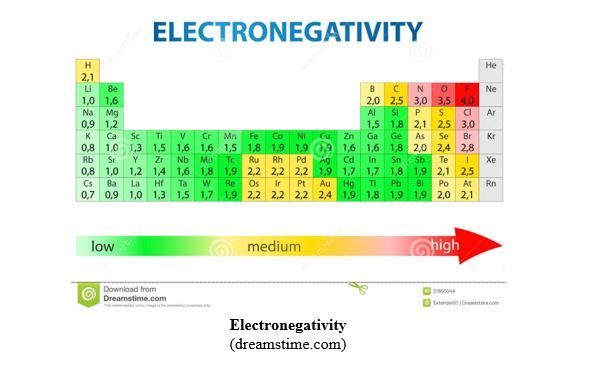
Electronegativity is the capacity of an atom participating in a chemical bond to attract towards itself the cloud or the bonding pair of electrons. It is a relative value and Fluorine (F), the most electronegative chemical element, is taken as reference. F has been attributed the highest value of EN = 4.0, according to Pauling Electronegativities. Caesium (Cs) is the least electronegative chemical element with EN = 0.7. No EN is attributed to Group 18, the rare gases, because of their tendency not to form chemical bonds. Generally, non-metal elements, due to their high effective nuclear charge, have higher EN values compared to metals
N.B: Sometime people have confusion between Electronegativity and Electron Affinity. EA is related to an isolated gaseous atom, whereas EN is related to an atom involved in a chemical bond.
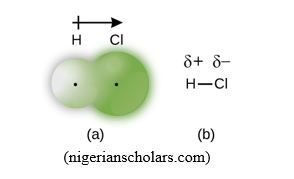
F. Polar covalent bond
A polar covalent bond is a covalent bond where the sharing of bonding pair of electrons is not equitable. This happens in principle when two different atoms, hence with different EN, participate in the formation of the chemical bond. When this occurs, the more electronegative atom attracts more the bonding pair of electrons and this creates a partial charges on the two atoms, negative partial charge, ∂-, on the more electronegative atom, and positive partial charge on the less electronegative atom. The bond formed is then called Polar Covalent Bond, example, H∂+Cl∂-
In principle it has been agreed that any chemical bond between two different atoms, with ΔEN ≤ 1.7 is considered as covalent polar, and ΔEN > 1.7 as ionic (see Part 4); HCl(ΔEN= 0.9) and HF(ΔEN = 1.9) are examples of polar covalent and ionic bond respectively. But for simplification, the chemical bonds where ΔEN is very small are considered as simply covalent. Example: C-H (ΔEN = 0.4) bond is considered as simply covalent, because ΔEN is negligible.
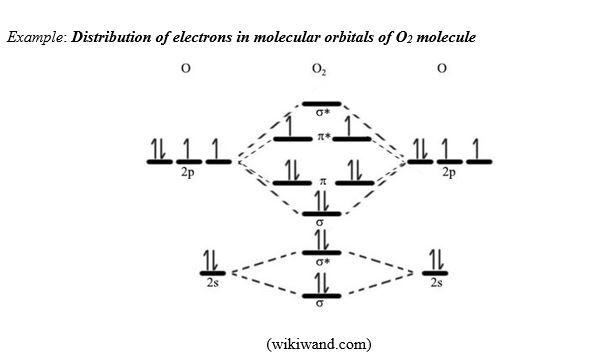
G. Molecular orbital theory and bond order
The concept of molecular orbitals or Molecular orbital theory is based on Quantum Mechanics Theory. In that theory, when two wave functions are added, they do it in two ways: they do it in-phase, and out-of-phase. In-phase addition stabilizes the molecule, whereas out-of-phase addition makes the molecule less stable.
For instance s-s atomic orbital overlapping gives two sigma molecular orbitals, one bonding (σ), the other anti-bonding (σ*).
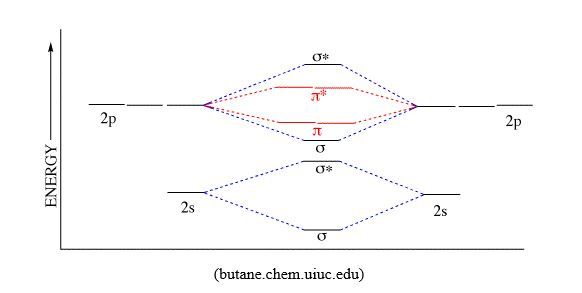
When p orbitals overlap, there is one Head-to-head overlapping that forms one bonding σ molecular orbital and one antibonding σ*molecular orbital, and two Side-by-side overlapping that gives two bonding π molecular orbitals and two antibonding π* molecular orbitals (see diagrams below)
As you can notice, the result is that antibonding orbitals have higher energy than bonding orbitals, and π orbitals have higher energy than σ orbitals.
When building the electron structure in a molecule, the total number of valence electrons from both atoms is distributed into the molecular orbitals in accordance to the same rules to build the atomic electron configuration: Aufbau Principle, Pauli Exclusion Principle, and Hund’s rule.
When building the electron configuration in molecular orbitals, take into consideration that in terms of energy: σs < σs*<σp<π < π* < σp*, where the indices represent the atomic orbitals combined.
When you compare the results of the electrons structures of molecules obtained by using the Lewis theory to the ones obtained by using the molecular orbital theory, you notice the following:
- In some cases, the two theories give the same results; for example H2 and N2 molecules. In H2 and N2 molecules, according to both theories, all valence electrons in the molecules are paired.
- In some other cases, the two theories gives different results. For instance in O2 molecule. Lewis structure shows that all valence electrons are paired, whereas the molecular orbital theory shows presence of unpaired electrons (see diagram of O2 molecule above)!
Experimental results have shown that O2 is paramagnetic; this confirms the structure obtained using molecular orbital theory in which there are 2 unpaired electrons responsible for the observed paramagnetism.
Bond order: the molecular orbital model allows us to calculate the bond order. The bond order indicates the stability of a chemical bond between 2 atoms. A simplified mathematical expression to calculate the bond order is:
BO = (Nb – Na)/2, where Nb = number of electrons in bonding orbitals, and Na = number of electrons in anti-bonding orbitals.
In the examples above: H2 (BO = 2/2 = 1); N2 (BO = 6/2 = 3); O2 [BO = (6-2)/2 = 2]. From this we can predict that O2 molecule is more stable than H2 molecule, but less stable than N2 molecule.
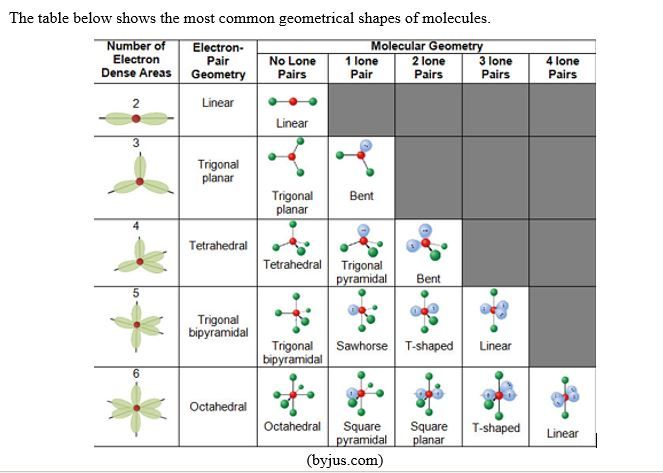
H. Shape of molecules and VSEPR Theory (Valence Shell Electron-Pair Repulsion)
Lewis structures are planar structures, i.e. bidimensional structures. Yet it has been proven that many molecules are tridimensional! Even for planar molecules, Lewis doesn’t explain why some forms are preferred to others; example, linear structure vs bent structure.
The VSEPR Theory tries to answer to that problem. It is based on the assumption that a molecule adopt a geometrical structure in which the repulsion between valence bonding and non-bonding electrons of the atoms in the molecule are minimized.
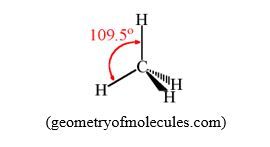
To illustrate this, let’s take the example of CH4 molecule. Let’s imagine that the molecule CH4 is square planar; the angle between two adjacent C-H bonds will be 90O. This is not the best structure to minimize the repulsion between bonding pairs of electrons. But if the molecule adopts a tetrahedral shape, the angle between two adjacent C-H bonds will be 109.5O (tetrahedral angle); that will minimize the repulsion more than the square planar structure.
In this concept, the repulsions:
lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair.
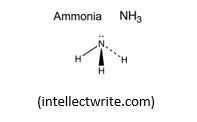
NH3: this molecule, according to VSEPR Theory, should adopt a tetrahedral shape. But the repulsion between the lone pair on N, is greater than the repulsion between bonding pairs in N-H bonds. This results into a pyramidal shape or a deformed tetrahedral shape with an angle between adjacent N-H bonds of 107.8o, instead of the tetrahedral angle of 109.5o found in CH4. Therefore the shape of Ammonia molecule is Trigonal pyramidal, a pyramid with a triangular base, i.e. a deformed tetrahedral shape.
H2O: according to the same theory, the molecule H2O would be linear as shown below.
Although VSEPR theory has helped in explaining or predicting the shapes of a certain number of molecules, it has its own weaknesses, among others:
- VSEPR theory is qualitative, not quantitative.
- VSEPR predicts that halides of Group 2 metals will have a linear structure, yet their actual structure is a bent one.
- As shown above, VSEPR theory predicts that a molecule such as H2O is linear yet we know that H2O is a bent molecule!
I. Hybridization of atomic orbitals
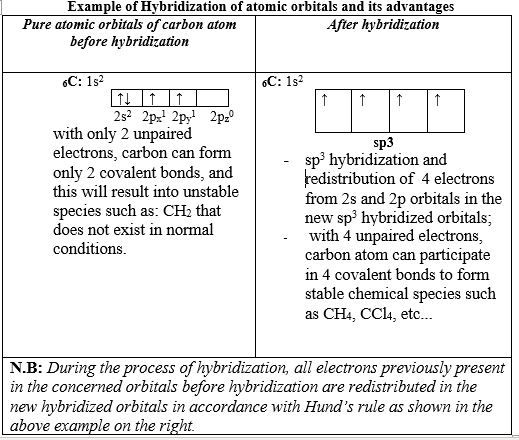
The difficulty experienced in predicting or explaining many molecular shapes led to a new concept: Hybridization of atomic orbitals.
When pure atomic orbitals (s, px, py, pz, etc.) are used in the formation of covalent bonds, some covalent molecules and shapes cannot be explained.
For example, according to the electron configuration of 6C: 1s22s22p1p1p0, carbon atom can only form 2 covalent bonds, using its 2 unpaired electrons in p orbitals, such as CH2, CCl2, etc. But those species don’t exist, but CH4 and CCl4 are known compounds!
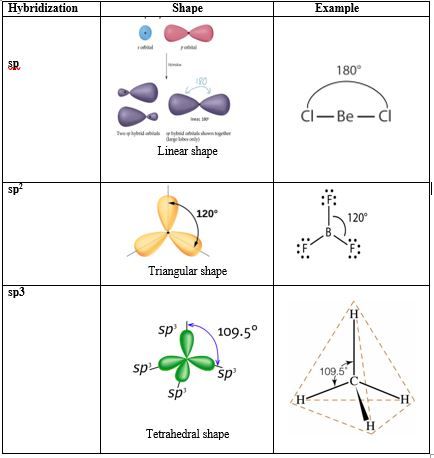
To overcome that challenge, the concept of Atomic Orbital Hybridization was introduced by Linus Pauling. Pauling proposed that atomic orbitals can be combined to form new atomic orbitals called: Hybrid orbitals with different orientations and shapes such as:
One s + one p → 2 new orbitals called sp hybridized orbitals: linear shape
One s + two p → 3 new orbitals called sp2 hybridized orbitals: triangular shape
One s + three p → 4 new orbitals called sp3 hybridized orbitals: tetrahedral shape
This concept of atomic orbital hybridization is useful to explain the shapes of molecules made of three or more atoms.
The hybridization process consists in combining a certain number of pure atomic orbitals and redistribute the total of electrons, present in the pure orbitals concerned.
In the table below, the example of sp3 hybridization of carbon is given, that results in the formation of the tetrahedral CH4molecule.
The advantage of the concept hybridization of atomic orbitals in comparison to the VSEPR theory is that the hybridization of orbitals is mathematically established; i.e. orbitals are solutions of a mathematical equation, the Schrodinger Equation. Hybridization of atomic orbitals helps to explain some shapes that VSEPR theory cannot explain, such as for example the bent shape of H2O molecule,
Common shapes of hybridized atomic orbitals
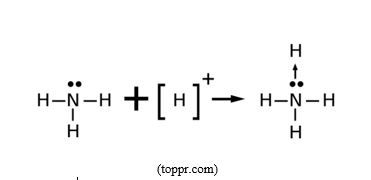
J. Coordination bond
A coordination bond is a covalent bond where the shared pair of electrons is given by one of the two participating atoms which acts as electron donor, whereas the other atom acts as electron acceptor or electron deficient. This bond is expected to be polar covalent because it is formed between to different atoms with different electronegativities.
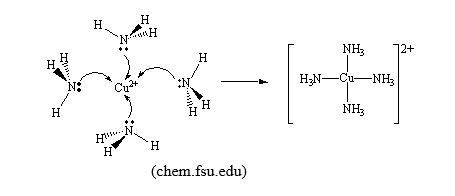
In this example, the pair of electrons shared between nitrogen, electron donor, and hydrogen ion, electron deficient/electron acceptor, is given by nitrogen atom from ammonia molecule, H3N:, to be shared with H+ ion. Coordination bond is found in coordination compounds or complexes formed between transition metal cations, electron deficient, such as Cu2+ion, and ligands, donor of pair (s) of electrons, such as NH3.
Although a coordination bond is given that special name, it is a covalent bond, the only difference is that the shared pair of electrons is given by one atom participating in the bond. But once formed, there is no way a coordination bond can be distinguished from an ordinary covalent bond.
K. Characteristics of a Covalent bond: Strength, Length, Vibration and Bond dissociation energy, solubility of covalent compounds
We have seen that a covalent bond results from the attraction force between the bonding electrons and the two nuclei that participate in the bond.
1. Strength of a chemical bond: is the force of attraction between the bonding electrons and the two nuclei of the atoms which form the bond.
The bond will be strong or weak depending on that force of attraction. Some factors affect the strength of a chemical bond:
- The volumes of the participating atoms determine the distance between the two nuclei, but also the distance between the bonding electrons and the nuclei. Small atomic volumes result in stronger attraction between the nuclei and the bonding electrons; this results in a Strong chemical bond. Whereas large volumes result in weak attraction between the nuclei and the bonding electrons, resulting in a weak chemical bond.
- Therefore the strength of a chemical bond is reflected in its length: Strong bonds are shorter than Weak bonds.
- Multiple bonds: bring more bonding pairs of electrons between the two nuclei, increasing the attraction between bonding electrons and the two nuclei, hence a multiple bond is shorter and stronger than a simple bond.
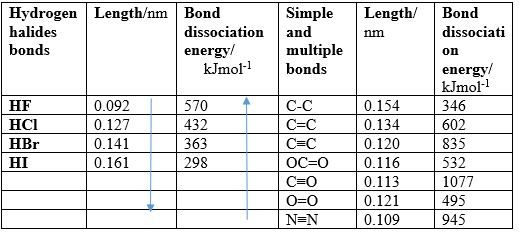
2. Bond length: it’s the average distance between the nuclei of the two atoms that participate in bonding.
3. Bond dissociation energy: minimum energy required to break a chemical bond homolytically, i.e. so that each atom remains with one electron of the bonding pair. Strong bonds have high bond dissociation energy, whereas weak bonds have low dissociation energy. Bond dissociation energies vary about 150-500 kJ/mol.
4. Vibration: chemical bond is not rigid; it acts as a spiral spring between the two atoms and vibrates continuously (except at Absolute zero). Vibration is a periodic back-and-forth motion of the particles of an elastic body. And the bond length is the average distance between the nuclei.
5. Solubility: the solubility of a chemical compound in a solvent depends on the chemical bonds of the chemical compound and the solvent.
- Non-polar covalent compounds are generally insoluble in water, a polar solvent.
- Polar covalent compounds are generally soluble in water and in polar organic solvent.
(we will see more details on that concept in another post)
Observations:
- Increasing of bond dissociation energy with decreasing of bond length,
- Increasing of bond dissociation energy with bond multiplicity (single, double, triple), and
- Decreasing of bond length with bond multiplicity.