Nuclear radiations bear energy. Depending on their energy, mass and charge, they can penetrate or be stopped by matter, and this is called penetration power. The figures below illustrate this:
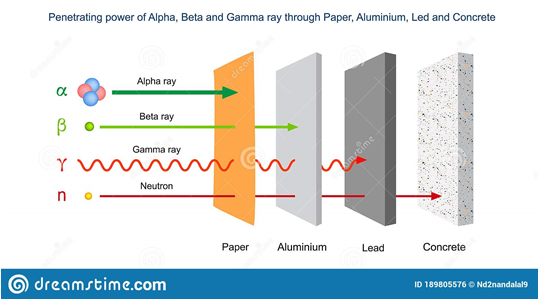
(dreamstime.com)
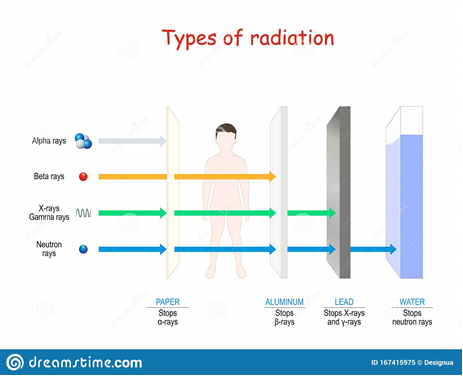
(cheggy.com)
Few explanations on the causes of the penetration power of some Radiations:
- Alpha rays (α, 42He++): less penetrating for the following reasons: are stopped easily in their penetration due to their high mass, and their positive charge
- Beta rays (β, 0-1e): more penetrating than alpha rays because of their zero mass
- Gamma rays (γ): more penetrating than beta rays; they have no mass and no charge,
- Neutron rays (n): most penetrating rays: no charge and highly energetic.
Ionization power of nuclear radiations
Ionizing radiation is a form of energy that acts by removing electrons from atoms and molecules of materials that include air, water and living organisms.
All types of nuclear radiation are ionizing radiations; Alpha radiation is the most ionizing due to its mass and high energy, whereas Gamma radiation is the least ionizing.