Explain the concepts of:
- The rate of a chemical reaction
- The rate-determining or rate-limiting step of a chemical reaction
- The difference between the “limiting reactant” concept and the “rate-limiting step” concept.
Solution:
Introduction: What a balanced chemical equation tells us and what it doesn’t tell us!
A balanced chemical equation shows the starting point, i.e. the reactants and the end point, i.e. the products. The chemical equation alone doesn’t show or tell if a reaction is fast or slow, if a reaction will take place or not and what happens between the starting and end points.
In Problems #17 and #18, we have seen the conditions or factors that allow us to conclude if a chemical reaction represented by a chemical equation will take place or not.
In the next paragraphs, we’re going to see other properties of a chemical reaction.
- The Reaction Rate
Any change process takes time. It is the same for chemical reactions, chemical reactions can take a range of time from very fast, a fraction of second to a very long time or several years.
Take an example, if you burn a piece of paper, it will take few seconds or minutes to burn completely. But if you leave a piece of iron outside, it will take many years to be transformed into iron (III) oxide/rust.
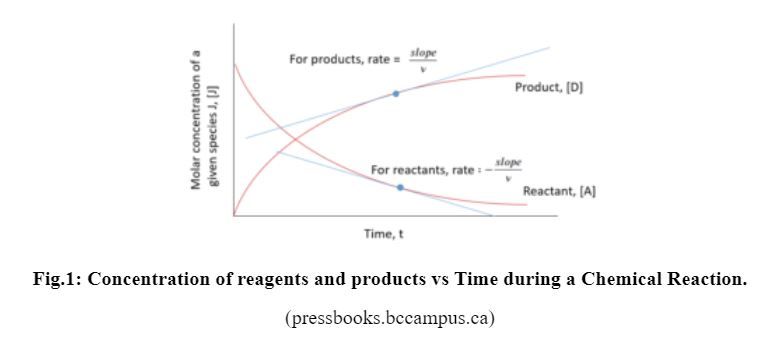
When a chemical reaction takes place, the reactants are consumed, i.e. their amounts decrease, whereas the products are formed, i.e. their amounts increase. The amounts can be expressed in terms of concentrations as shown in the Figure below.
If you examine the two curves in the Fig. 1 , you notice that the slopes of their tangent lines decrease as the reaction proceeds and the slope of the tangent line to the curve at any given point of the curve represents the change of concentration with time, or the rate of the reaction at that point.
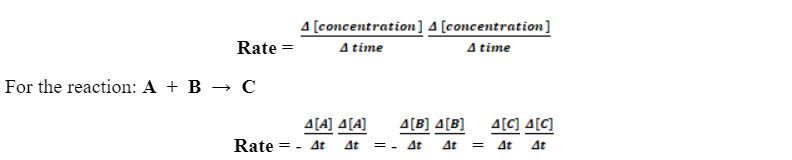
The reaction rate, also called the speed of the reaction, is expressed as the change of concentrations of the reactants or products with time. In other words, it is the decrease of the concentration of a reactant (which is consumed), or the increase of the concentration of a product (which is formed), per unit time as shown above.
The change of the concentrations of the reactants with time has a negative sign (-) because their concentrations decrease with time, whereas the change of the concentrations of the products has a positive sign because their concentrations increase with time.
The reaction rates are reported either as the average rate over a period of time or as the instantaneous rate at a single time. They are differential functions.
Reaction rates generally decrease with time because the reactant concentration decreases as reactants are converted into products. And the reaction rates generally increase when reactant concentrations are increased.
Rate law and Rate Constant
A rate law is an expression that relates the rate of a reaction to the rate constant and the concentrations of the reactants.
Rate Law = k[A]m[B]n
Where k is the rate constant, which is a proportionality constant for a given reaction, m and n are the orders of the reaction relative to reactants A and B respectively.
N.B: m and n are not to be confused with the coefficients in the chemical equation; they may be a whole number (not necessarily equal to the equation coefficient), or a fractional number.
The rate law is a mathematical description of experimentally verified data. The rate law is an integrated function.
As seen, the rate law is dependent on the concentrations of the reactants, however there are also other factors that can influence the rate of reaction, such as temperature and catalyst.
N.B: For more details about the reaction rate and rate law you can consult a Chapter of Chemistry related to “Mechanisms of Chemical reactions”.
2. The Rate-determining or Rate-limiting step of a chemical reaction
The chemical equation can be compared to a Relay Race where two photos are taken: one showing all athletes at the starting line (the part of the equation at the left of the arrow), and another showing athletes at the finishing line (the part of the equation at the right of the equation).
But, what happened between those two points?
This can also be compared to an industrial production where you have the starting point made of raw material, and the output of the production made of the products.
In both examples, between the starting and the end points, something happens; there are steps:
Industrial production:
Entry Point (Raw Material) >> Step1>>Step2 >>Step3 >>...Output (Final Product).
Chemical reaction:
Entry Point (Reactants A + B) >>Step1 >> Step 2 >>...Output (Products C + D).
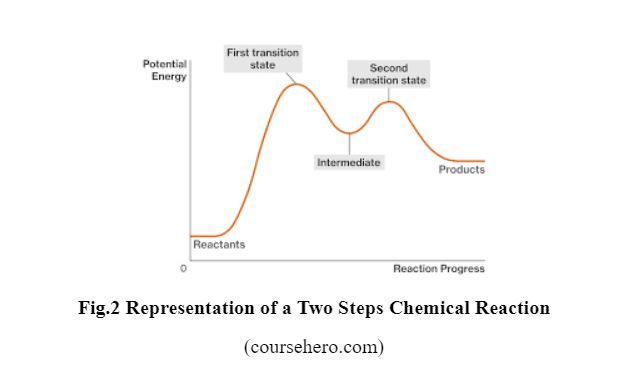
The chemical equation shows only the beginning and the end of the reaction, but many chemical reactions pass through more than one step, called “Elementary Steps” as shown in the figure below.
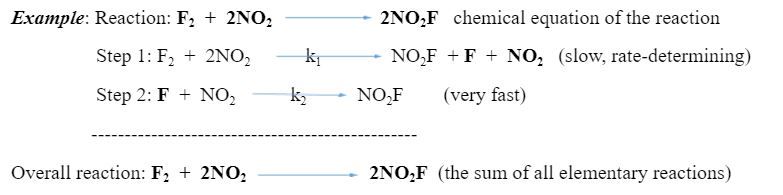
Each Step has its activation energy, Ea (see Problem 18), and its rate. But the slowest step, i.e. the step with higher Ea, is the one that influences the speed/rate at which the overall reaction proceeds; it is called the “rate-determining or rate-limiting step.” In Figure 2 above, Step 1 with higher Ea is the rate-determining step.
N.B: The activation energy of a chemical reaction is closely related to its rate. Specifically, the higher Ea, the slower the chemical reaction will be. This is because molecules can only complete the reaction once they have reached the top of the Ea barrier.
k = Ae-Ea/RT; this is the Arrhenius equation in the collision model of chemical kinetics showing the relationship between the rate constant k and the activation energy, Ea; A= frequency factor.
The rate-determining step can be compared to the neck of a funnel. The rate at which water flows through the funnel is limited/determined by the width of the neck of the funnel and not by the rate at which the water is poured into the funnel. Like the neck of the funnel, the slowest step of a reaction determines the rate of the reaction.
The same as in the example of a relay race, the overall speed of the team will be limited by the slowest team member.
Rate law of the reaction = k1[F2][NO2]2 = the rate law of the slowest step
3. Rate-limiting step vs limiting reactant
Although rate-limiting step and limiting-reactant sound almost the same, they are very different. The limiting reactant is the reactant whose quantity limits the extent of the chemical reaction, when the quantities of reactants are not in stoichiometric ratios. Once the amount of the limiting reactant is finished, the reaction ends.
Whereas the rate-limiting step is the slowest step that limits the overall rate of the reaction.
The limiting reactant affects the extent and the amount of the products of a reaction; whereas the rate-limiting reactant affects the rate of the reaction.