In problem 10 part II, paragraphs a) to g), we have talked about Covalent bonds. We saw Sigma (σ) and Pi (π) bonds and their molecular orbitals. Those bonds are the most common, particularly in Organic Chemistry. But there exist other less common types of covalent bonds such as Delta (δ) bonds.
The notation Delta molecular orbital (δ) and Delta bond was introduced by Robert Mulliken in 1931. Delta bond (δ) is a chemical bond that involves delta (δ) molecular orbitals. It results from an overlapping of d-orbitals on two different atoms and resembles a d-orbital when viewed along the axis of a molecule; it has two units of orbital angular momentum around the internuclear axis with two nodal planes which contain the internuclear axis and go through both atoms as shown below.
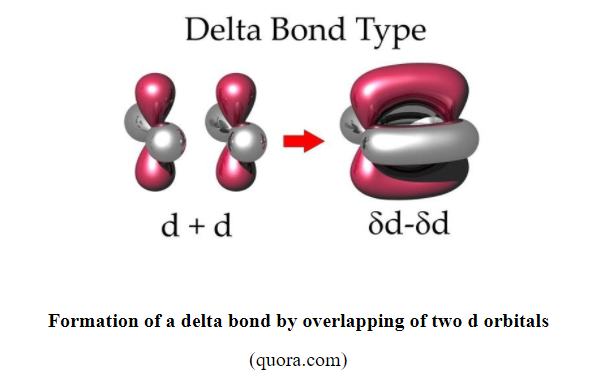
Delta orbitals contribute to the bonding in organometallic species of transition metals, and in cluster compounds of transition metals such as [Re2Cl8]2- with a quadruple bond. The first compound identified as having a δ bond was potassium octachlorodirhenate(III) by F.A.Cotton, in 1965.
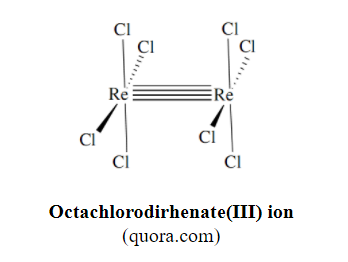
The above quadruple bond is described as σ2π4δ2 meaning: one Sigma bond with 2 electrons, two Pi bonds with a total of 4 electrons, and one Delta bond with 2 electrons.