Metallic Bond
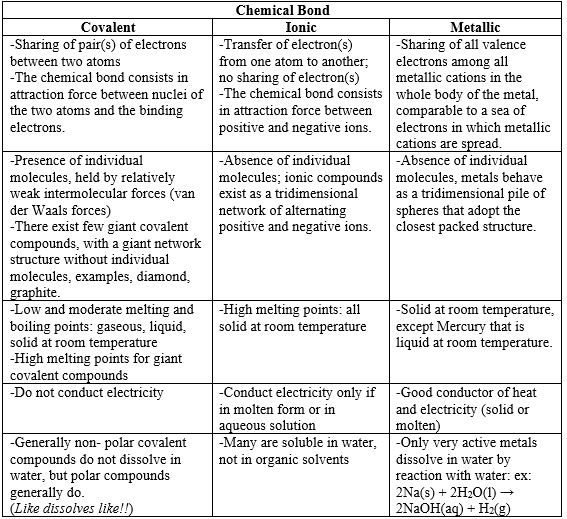
Contrary to the covalent bond where there is sharing of pairs of electrons between 2 atom nuclei, in metallic bonding, all atoms in the whole body of the metal put together their valence electrons. The metallic bond then results from the attraction force between the pool of electrons, compared to a sea of electrons, and all metallic cations spread in the pool of electrons (Fig.5). In metallic bond, the bonding electrons are not localized between any two atoms, they are mobile or delocalized and can move in the whole body of the metal. This last property explains why metals conduct electricity and heat.
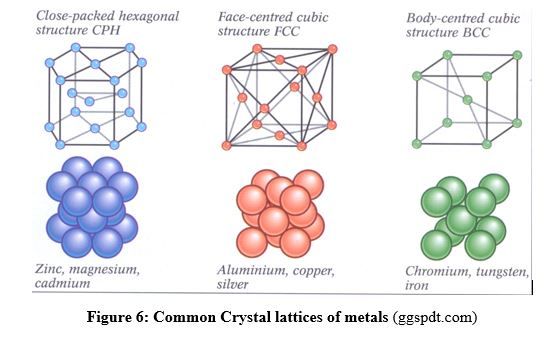
The structure of a metal crystal is called “close-packed” or “close-packing” structure, where each atom has high coordination number (lots of neighbors). This can be compared to the situation where equal spheres or equal oranges (or apples) are filled in a box or stacked on a shelf (Fig.6). That structure explains also why metals are malleable and ductile because plans of atoms can slide one over another.
The chemical symbol to represent a given metal is the same as the chemical symbol of the atom. Example Sodium metal (Na), Iron (Fe), Gold (Au), etc.
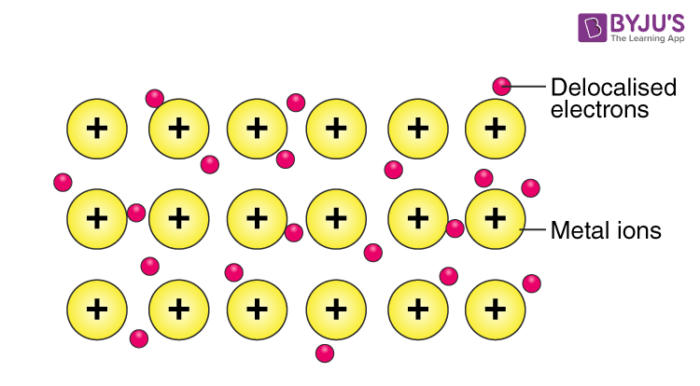
Figure 5: Metallic Bonding
Source: byjus.com
Characteristics of metallic bond
The bond is generated by the force of attraction between all the metallic cations and all valence electrons that form a kind of sea of electrons. The concept of bond length is irrelevant in metallic bond because the bond is not created between two atoms or two ions.
A metal that has many valence electrons and highly charged cation will have stronger metallic bond than the one that has few valence electrons and cation with small positive charges.
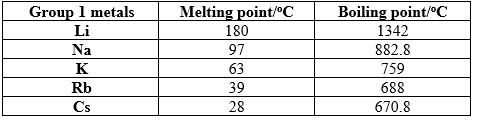
This explains why transition metals are generally harder than alkali and alkali-earth metals. This also explains why, for example in group 1 metals, hardness of metals decreases down the group. As you go down the group the atomic volume increases due to the increasing of the atomic radius. As the atomic radius increases, the attraction between the valence electrons and the cations decreases, leading to weaker metallic bonds. So, as you go down a group, the metals softness increases and the melting point decreases down the group.
Properties of metals
Due to the characteristics of metallic bond described above:
- Metals, in general, have high melting point and are solid at room temperature, except Mercury (Hg) which is liquid. Gallium metal (Ga) is solid at room temperature but has a low melting point (30oC), i.e. it will melt in your hands.
- They conduct heat and electricity.
- They are, malleable (can be beaten into different shapes) and ductile (can be pulled into thin wires).
- Freshly cut surface of metals is shiny
(N.B: Contrary to the rare gases that exist as isolated atoms and are represented by their chemical symbols (example: He), metals, as substances, are represented by their chemical symbols (ex: Na) although they do not exist as isolated atoms. Metals also do not exist as individual molecules but as giant tridimensional close-packet structure.)
Summary on Chemical Bond and Properties
SEE YOU NEXT WEEK!