Intermolecular Forces
Up to now we have discussed about chemical bond or the force that binds atoms together within a molecule, that force is also called intramolecular force.
The force that holds molecules together in a covalent pure substance or compound is called intermolecular attraction force (otherwise the entire universe would be gaseous!). Those intermolecular forces are called “Van der Waals forces”. They are weaker than covalent bonds.
A- Van der Waals Forces
These are forces that hold together molecules in a covalent compound due to the dipole-dipole attraction. There are many kinds of these interactions:
- Dipole-dipole attraction: attraction between two permanent dipole molecules:
ex: H∂+-Cl∂-<-->H∂+-Cl∂- - Dipole-induced dipole attraction: attraction between a permanent dipole molecule, such as NH3 and a non-polar molecule, such as I2. The permanent polar molecule induces a dipole in the other molecule:
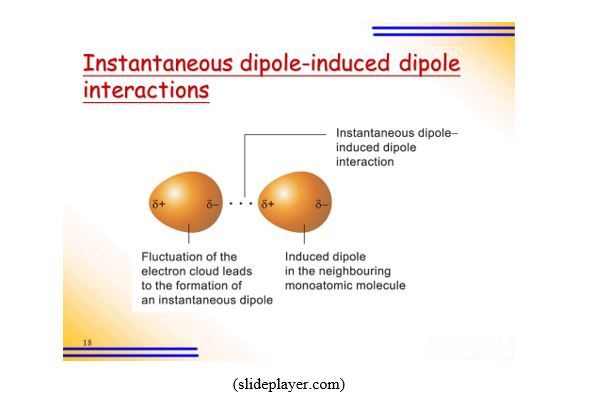
ex: (N∂-H3∂+, I-I) → N∂-H3∂+<-->I∂--I∂+ - Instantaneous dipole-induced dipole or London Dispersion forces (LDF): this occurs between two atoms or non-polar covalent molecules. It is a temporary attraction force that results when the electrons in two adjacent atoms or non-polar covalent molecules reorganize to occupy a position not symmetrically distributed around the nucleus, making the atoms or molecules temporary dipoles.
Comparison of Van der Waals forces: dipole-dipole > dipole-induced dipole > instantaneous dipole-induced dipole (LDF)
B- Van der Waals Forces and Physical Properties of Compounds
These intermolecular forces which make compounds at room temperature, covalent substances or compounds, are:
- Gaseous: absence or almost absence of Van der Waals forces.
Ex: Hydrogen (H2), Carbon dioxide (CO2), Methane (CH4), Chlorine (Cl2). - Liquid: presence of weak Van der Waals forces.
Ex: Water (H2O), Ethanol (CH3CH2OH), Pentane (CH3CH2CH2CH2CH3). - Solid: presence of strong Van der Waals forces.
Ex: Sugar/Glucose (C6H12O6), Iodine (I2).
Intermolecular forces are responsible for the melting and boiling points of covalent compounds. Covalent compounds, except few, are also called molecular compounds or substances because they are made of individual molecules held together by intermolecular forces of van der Waals. That is why they have generally low boiling and melting points.
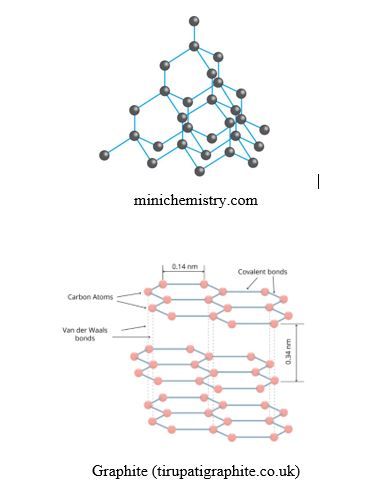
Diamond and Graphite are covalent substances but do not possess individual molecules:
- Diamond consists in a giant tridimensional network of carbon atoms bonded by covalent bonds, where each carbon atom is bonded to 4 other carbon atoms in a tetrahedral structure; this gives to the diamond the property of being the hardest natural material (10 Mohs). It is used in cutting instruments. It melts at 4,027oC
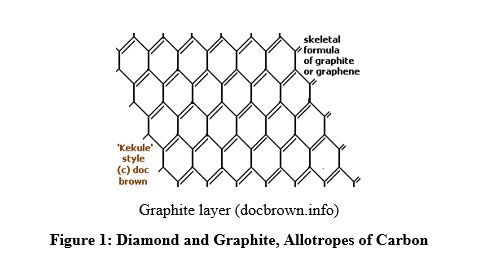
- Graphite consists in layers made of hexagonal structures of carbon atoms; the layers are superimposed one over another and held by weak van der Waals forces; this explains why graphite is soft and can be used as lubricant or in pencils. It melts at 3,600oC. Graphite conducts electricity due to the presence of mobile electrons in the benzene rings of graphite layers, due to the presence of delocalized or conjugated pi bonds in the rings of the graphite layers.
C- Van der Waals Forces and Solubility:
Van der Waals forces play an important role in the solubility of substances in different solvents (to be seen in another post)
D- Hydrogen bond
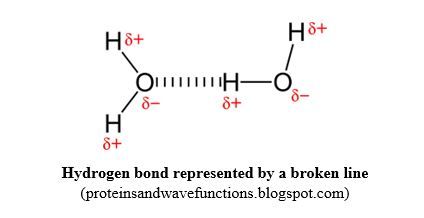
Hydrogen bond is an intermolecular force between molecules or parts of molecules having the following groups: O-H, N-H and H-F. It is a special Van der Waals force.
If you look at those 3 groups, you notice that they are made of polar bonds between Hydrogen (EN = 2.1), and the most electronegative elements of the periodic table, O (3.50), N (3.0) and F (4.0). The consequence is that the chemical bonds formed between hydrogen and those atoms, are highly polar. And the attraction force between those groups from different molecules is strong compared to other intermolecular forces; that is why it has been given a special name of hydrogen bond.
Because hydrogen bond is a strong force compared to other van der Waals forces, this explains why the presence of hydrogen bond in a compound gives to that compound special properties in comparison to others compounds of the same family where there is no hydrogen bond. Those properties are for instance, high melting and boiling points, high solubility in water, etc.
Examples:
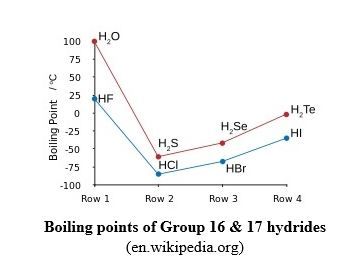
- The abnormally high boiling point of H2O (Mm = 18): 100oC, compared to the boiling point of H2S (Mm = 34): -60.7oC.
- The abnormally high boiling point of HF (Mm = 20): 20oC, compared to the boiling point of HCl (Mm = 36.5): -85oC.
Normally melting and boiling points increase with increasing of molecular mass (see the graph below)!
The graph above shows the abnormally high boiling points of water (H2O) and hydrogen fluoride (HF), where hydrogen bond is present, compared to the other hydrides in the same families.
Without hydrogen bonding in water, water would boil at about -70oC! (There would be no liquid water on Earth! Can you imagine the Earth without water?!)
Hydrogen bond is responsible for holding the two chains in the double helical structure of DNA together.
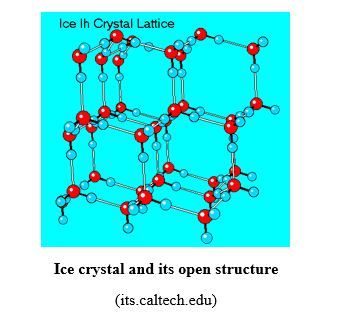
Hydrogen bond is responsible for the open structure of ice, as shown in the figure below. That leaves a lot of empty space (see fig. below), due to the stiffness, rigidity and non-flexibility of hydrogen bonds at low temperature, and makes ice less dense than liquid water. That is why ice floats on water.
In liquid water, hydrogen bond is flexible and allows the molecule to be more packed, without lot of empty space, making it denser than ice and the latter floats on liquid water (see fig. below).
E- Properties of Covalent Compounds
As seen, covalent compounds are in two categories:
1. Molecular covalent
- Molecular covalent compounds are made by individual molecules held together by Van der Waals forces or hydrogen bond. They are gases, liquids or solid with relatively low melting points.
- They do not conduct electricity.
- Most of them are not soluble in water. But for those that dissolve in water, their solutions do not conduct electricity.
2. Giant covalent
- Giant covalent substances or compounds, such as diamond and graphite, have high melting points.
- They are generally poor conductor of electricity, except graphite which is a good conductor of electricity.
- They are not soluble in water.