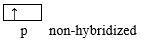Notice: The following problems 11 to 14 are direct application of the notions and principles developed in problem 10. I would advise you to do them, by yourself or with colleagues, in order to evaluate how deep your understanding of those notions and principles is.
1.
Explain the difference between valence electrons and valence (valency) of an atom. In what aspects of chemistry is knowing the valence electrons and the valence of an atom useful or can be used?
2.
Draw a Lewis structure of CO and HCN
3.
Using the concept of Molecular orbitals:
i) Draw the electronic structure of N2 molecule,
ii) Calculate the bonding order of N2 molecule,
iii) Say, if N2 molecule is diamagnetic or paramagnetic and justify your answer,
iv) Predict if He2 molecule can exist or not and justify.
4.
Using the concept of orbitals, explain how the following molecules are formed (the information given about each molecule will help you in answering to the question).
- H2O: bent molecule, inter-bond angle of 104.5o and 2 lone pairs of electrons on O atom,
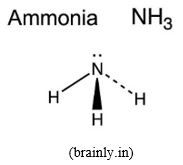
- NH3: pyramidal molecule, inter-bond angle of 107O and 1 lone pair of electrons on N atom,
- C2H2 or H-C≡C-H,
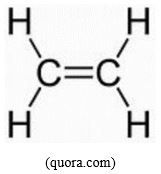
- C2H4 or CH2=CH2, angle between C-H bonds is 120o
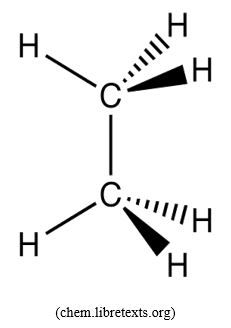
- C2H6 or CH3-CH3: angle between C-H bonds is 109o
Solution:
1.
The definition of Valence Electrons has been given in a previous problem.
Here is the definition of the valence:
(i) The valence (valency) of an atom or a radical is its combining power, which is equal to the number of hydrogen atoms that the atom or the radical can combine with or displace in a chemical compound(hydrogen has a valence of 1).
(ii) The valence can also be defined as the number of chemical bonds an atom will usually form.
Examples:
- 1 Oxygen atom can react with 2 Hydrogen atoms to form H2O molecule, hence Oxygen has a Valence of 2, in other words, can form 2 chemical bonds.
- 1 Chlorine atom can react with 1 Hydrogen atom to form HCl molecule, hence Chlorine has a Valence of 1, or can form only 1 chemical bond.
In covalent molecules, the valence of an atom is equal to the number of bonds formed by the atom:
Examples:
- In CO2 (O=C=O): O has a valence 2, whereas C has the valence 4.
- In CH3OH: H has the valence 1, C has 4, and O has 2.
When dealing with a molecule made of 2 different atoms, the sums of the valences of the atoms of the two elements must be equal.
Examples:
- CO2: C (4) and O (2x2 =4);
- NH3: N (3) and H (1x3= 3);
- PCl5: P(5) and Cl (1x5 =5).
In ionic compounds there are two parts:
- a cation, with a positive charge
- an anion with a negative charge.
Since the chemical formula of the ionic compound is neutral, this means that the total of positive charges and the total of negative charges must cancel each other.
Examples:
- In Na2S: Na+ (2x1+ = 2+), S2-(2-)
- In FeCl2: Fe2+(2+), Cl- (2x1- = 2-)
- In Fe2O3: Fe3+(2x3+ = 6+), O2-(3x2- = 6-)
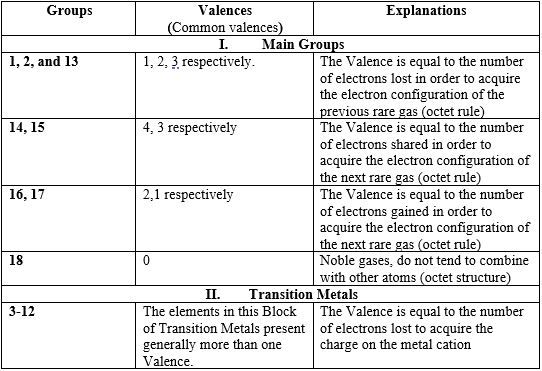
Common Valences across the Periodic Table
N.B:
- The valences shown in this Table are the most common of elements; but some elements may show more than one valence. For instance, in CO2 (O=C=O) molecule, C and O have valences (4) and (2) respectively; but in CO (C≡O) molecule, both C and O have valence (3).
- One characteristic of Transition Metals is that they present generally more than one valence (oxidation state).
- Some elements are known to have only one valence, for example Group 1 & 2 Metals are characterized by their only one oxidation state + or valence 1, and 2+ or valence 2 respectively. Whereas Group 17 Elements are known in most of their compounds with the oxidation state -1 or valence 1.
Importance of knowing the valence electrons of an atom: helps you to know the nature of an atom, i.e. to know if an element is a metal, non-metal, etc. This also allows you to predict the chemical behavior of the atom (see problem 8).
Importance of knowing the valence (valency) of an atom: knowing the valence of an atom, a radical or an ion allows you to determine the chemical formula of compounds.
2.
CO - number of valence electrons: 4 + 6 =10
Skeleton structure:
:֟C-Ö: no atom has an octet, then reorganize the electrons by sharing 1 pair of electrons from C
:C=Ö: here C needs 2 electrons; reorganize the electrons by sharing 1 pair or electrons from O
:C≡O: triple bond and 1 lone pair of electrons on C and O atoms
HCN - number of valence electrons: 1 + 4 + 5 = 10
Skeleton structure:
H-֟C-֟N:C and N have not achieved the octet; so one pair of electrons from N is shared with C
H-֟C=N: then one pair of electrons on C shared with N:
H-C≡N: single bond between H and C and triple bond between C and N with a lone pair on N
3.
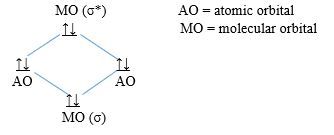
i) Molecular orbital diagram of N2
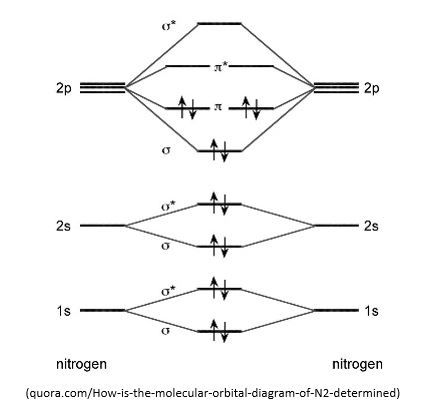
ii) Bonding order of N2: 6/2 = 3
iii) N2 is diamagnetic because all the electrons are paired
iv) He2:
He2cannot exist, because it has a bonding order of zero : (2-2)/2 = 0
4.
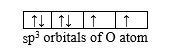
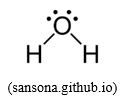
H2Ö: from the information given about that molecule, we can see that the shape of water molecule derives from a tetrahedral shape where 2 tetrahedral summits are occupied by lone pairs of electrons instead of hydrogen atoms. Since the repulsion of a lone pair of electrons towards the bonding pair of electrons is stronger than bonding pair-bonding pair repulsion, this explains why the inter-bond angle (104.3O) in water molecule is smaller than the tetrahedral angle (109.5O) found in a perfect tetrahedral shape such as CH4.
So, this shows that water molecule forms between oxygen atom and two hydrogen atoms, where O atom uses its sp3 hybridized orbitals to form σ-bonds with H, remaining with 2 lone pairs of electrons.
This explains why H2O molecule appears as a “bent molecular geometry”.
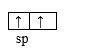
:NH3 from the information given about that molecule, we can also conclude that the shape of :NH3 molecule derives from a tetrahedral structure where one tetrahedral summit is occupied by a lone pair of electrons instead of a hydrogen atom. Here the central atom N uses its sp3 hybridized orbitals.
The same reasoning applies as for water molecule above. This results in a pyramidal shape with the inter-bond angle of 1070
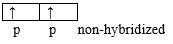
H-C≡C-H: the molecule being linear, this indicates that the two C atoms uses their sp hybridized orbitals. Each C atom uses one sp orbital to form a σ-bond with the other C atom, and the other sp orbital to form a σ-bond with H. The non-hybridized p atomic orbitals remaining on each C can overlap side-by-side, to form 2 Pi bonds. Therefore the triple bond is made of 1 σ-bond and 2 Pi-bonds.
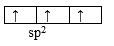
C2H4: according to the information given on the molecule, the central C atoms are using sp2 since each C is a center of a triangular structure. Each C can use the 3 sp2 hybridized orbitals to form 3 σ-bonds, 1 with the other C, 2 with H. The remaining un-hybridized p orbital on each C then overlap side-by-side with the other C to form a Pi-bond, and the result is what has been shown in the data of the problem.
C2H6: the information we have on that structure tell us that each C atom is the center of a tetrahedral. This implies that each C has used its sp3 hybridized atomic orbitals to form 4 σ-bonds; 1 with the other C atom and 3 between each C and H. This results in the structure described in the statement of the problem.
Don’t miss the next post!